|  | 
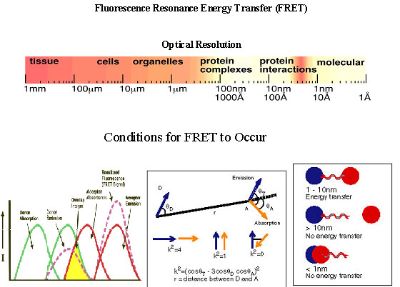
Fluorescence resonance energy transfer (FRET) is a process involving the radiationless transfer of energy from a donor fluorophore to an appropriately positioned acceptor fluorophore. FRET can occur when the emission spectrum of a donor fluorophore significantly overlaps (>30%) the absorption spectrum of an acceptor (see Figure 1), provided that the donor and acceptor fluorophores dipoles are in favorable mutual orientation. Because the efficiency of energy transfer varies inversely with the sixth power of the distance separating the donor and acceptor fluorophores, the distance over which FRET can occur is limited to between 1-10 nm. When the spectral, dipole orientation, and distance criteria are satisfied, illumination of the donor fluorophore results in sensitized fluorescence emission from the acceptor, indicating that the tagged proteins are separated by <.10 nm.
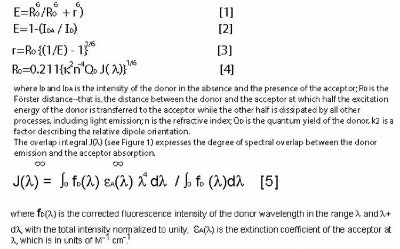
The energy transfer efficiency (E), the rate of energy transfer (kT), and the distance between donor and acceptor molecule (r) are calculated using the following equations:

Within the living cell, interacting proteins are assembled into molecular machines that function to control cellular homeostasis. These protein assemblies are traditionally studied using biophysical or biochemical methods such as affinity chromatography or co-immunoprecipitation. Recently, two-hybrid and phage-display methods have been used for detecting protein-protein interactions. These in vitro screening methods have the advantage of providing direct access to the genetic information encoding unknown protein partners. These techniques do not allow direct access to interactions of these protein partners in their natural environment inside the living cell, but using the approach of fluorescence resonance energy transfer (FRET) microscopy, this information can be obtained from single living cells with nanometer resolution.
FRET microscopy relies on the ability to capture weak and transient fluorescent signals efficiently and rapidly from the interactions of labeled molecules in single living or fixed cells. The occurrence of FRET signal (sensitized signal) can be verified by acquiring the two-emission signal bands of the double labeled cells excited with donor wavelength. If FRET occurs the donor channel signal will be quenched and the acceptor channel signal will be sensitized or increased. In principle, the measurement of FRET in a microscope can provide the same information that is available from the more common macroscopic solution measurements of FRET; however, FRET microscopy has the additional advantage that the spatial distribution of FRET efficiency can be visualized throughout the image, rather than registering only an average over the entire cell or population. Because energy transfer occurs over distances of 1-10 nm, a FRET signal corresponding to a particular location within a microscope image provides an additional magnification surpassing the optical resolution (~0.25 mm) of the light microscope. Thus, within a voxel of microscopic resolution, FRET resolves average donor-acceptor distances beyond the microscopic limit down to the molecular scale. This is one of the principal and unique benefits of FRET for microscopic imaging: not only colocalization of the donor- and acceptor-labeled probes within ~0.09 mm2 can be seen, but intimate interactions of molecules labeled with donor and acceptor can be demonstrated. Several FRET techniques exist based on wide-field, confocal and 2p microscopy as well as FRET/FLIM, each with its own advantage and disadvantage. All FRET microscopy systems require neutral density filters to control the excitation light intensity, a stable excitation light source (Hg or Xe or combination arc lamp; UV, Visible or Infrared lasers), a heated stage or a chamber to maintain the cell viability and appropriate filter sets (excitation, emission, and dichroic) for the selected fluorophore pair. It is important to carefully select filter combinations that reduce the spectral bleed through (SBT) to improve the signal-to-noise (S/N) ratio for the FRET signals.

The widely used donor and acceptor fluorophores for FRET studies come from a class of autofluorescent proteins, called Green Fluorescent Proteins (GFPs). The spectroscopic properties that are carefully considered in selecting GFPs as workable FRET pairs include: sufficient separation in excitation spectra for selective stimulation of the donor GFP, an overlap (>30%) between the emission spectrum of the donor and the excitation spectrum of the acceptor to obtain efficient energy transfer and reasonable separation in emission spectra between donor and acceptor GFPs to allow independent measurement of the fluorescence of each fluorophore. GFP-based FRET imaging methods have been instrumental in determining the compartmentalization and functional organization of living cells and for tracing the movement of proteins inside cells.
There are number of combination of FRET pair can be used depending on the biological applications. Selected popular FRET pair fluorophore are - CFP-YFP, CFP-dsRED, BFP-GFP, GFP or YFP-dsRED, Cy3-Cy5, Alexa488-Alexa555, Alexa488-Cy3, FITC- Rhodamine (TRITC), YFP-TRITC or Cy3, etc.

One of the important conditions for FRET to occur is the overlap of the emission spectrum of the donor with the absorption spectrum of the acceptor. As a result of spectral overlap, the FRET signal is always contaminated by donor emission into the acceptor channel and by the excitation of acceptor molecules by the donor excitation wavelength (see Figure 1). Both of these signals are termed spectral bleed-through (SBT) signal into the acceptor channel. In principle, the SBT signal is same for 1p- or 2p-FRET microscopy. In addition to SBT, the FRET signals in the acceptor channel also require correction for spectral sensitivity variations in donor and acceptor c hannels, autofluorescence, and detector and optical noise, which contaminate the FRET signal. How to correct the contaminated signals is explained in data process part.
|  |  |